Two precision clocks by Nicholson featured in Antiquarian Horology Journal
April 23rd, 2023share this

One of the most enjoyable things about researching Nicholson’s numerous interests and activities is the way that it has brought me into contact with so many people that I might never encountered otherwise. Experts in a variety of fields have been unbelievably kind and generous in sparing their time and energy to help me understand what Nicholson was up to and the context of his work at the time. One thing they all share has been a keenness to see that William Nicholson and his achievements should be better known and appreciated.
The most recent example is horological guru Jonathan Betts - Curator Emeritus at the Royal Observatory (National Maritime Museum), Greenwich, a horological scholar and author, and an expert on the first marine timekeepers created by John Harrison in the middle of the 18th century.
Jonathan is also the librarian to the Antiquarian Horological Society, and recently authored two articles about Nicholson which have been published in the society’s journal Antiquarian Horology, Vol 44, March 2023:
- ‘Two precision clocks by William Nicholson’; and
- ‘Notes from the Librarian: William Nicholson – so much more than a journalist.’
Forty years previously, in 1983, Betts had first encountered Nicholson (in regard to his comments on Explanations of Time-keepers Constructed by Mr Thomas Earnshaw and the late Mr John Arnold in 1806) and then in 2013 he acquired a collection of horological papers which had been published by Nicholson. A little while after this, I must have popped into his in-box after finding his paper on Nicholson’s good friend John Hyacinth Magellan (1722-1790).
In this article, Jonathan Betts forensically examines the two clocks which have been signed by Nicholson, although – as we know that Nicholson employed instrument-makers – ‘it seems very likely that Nicholson oversaw the clock’s construction to his specification’ rather than having any ‘hands-on’ involvement,
Nicholson’s table regulator clock lives at the British Museum (and was highlighted in an earlier blog). Betts describes the design and combination of features in the movement of this clock as “unique” and “evidently designed so that the technical features can be exposed and appreciated – very much in line with Nicholson’s desire to disseminate technical information”.
The second clock - featuring for the first time in public in the AHS Journal - is described as a ‘miniature gimballed regulator” with parts dated 1805 and 1806, and as “more elegant” and “even more exposed and ‘on show’”.
Unfortunately, Betts describes how this design had a ‘significant failing in the proportions of the dead-beat escapement’ which can lead to a stopping or bottoming, although this may have been prevented by the gimbal arrangement in the design.
A gimbal is a pivoted support that permits rotation of an object about an axis (for example, like a stabiliser on a hand-held camera), and it seems that this clock was ‘almost certainly mounted on a tripod and was thus intended to be used in a ‘portable’ environment’ for example, at sea or when surveying.
Could Nicholson have designed this for use this onsite at the West Middlesex Waterworks in late 1806?
To purchase Antiquarian Horology Volume 43
If you are interested in horology and the technical details of these two clocks, then it is possible to purchase a single issue of Antiquarian Horology Volume 43 via the AHS website Shop for just £8.50 plus P&P.

#41
'Experiments and Observations Made with Argand’s Patent Lamp,' - Shining a light on Nicholson's concentric wicks
July 18th, 2021share this

In The Collected Letters of Erasmus Darwin, edited by Desmond King-Hele, Letter 86-6 from Erasmus Darwin to Josiah Wedgwood, 21 April 1786, starts off “Sir, Mr Nicholson is an ingenious and accurate man …” and continues a series of discussions between the men about oil lamp designs, leading to the comment that “The pyramidical lamp would be more pleasing to they eye than the concentric one of Mr Nicholson.”
Having been searching for Nicholson’s 'concentric lamp' for many years, I was delighted to finally track it down in The London Magazine, of May 1785:
Experiments and Observations Made with Argand’s PatentLamp.
Sir
As the attention of the world has been much excited by the powerful effects of Argand’s Lamp, and as there are many who are desirous of making use of it provided its advantages were clearly ascertained, I presume the following description of the instrument and its effects will not be unacceptable to the public.
Yours, &C.
N.
The apparatus consists of two principal parts, a fountain to contain the oil, and the lamp itself. Of the former it is unnecessary to speak: the lamp is constructed as follows. The external parts consist of an upright metallic tube one inch and six-tenths in diameter, and three inches and a half in length, open at both ends. Within and concentric to this is fixed another tube of about one inch in diameter, and nearly of equal length; the space between these two tubes being left clear for the passage of air. The interior tube is closed at the bottom, and contains another similar tube a little more than half an inch in diameter. The third tube is soldered to the bottom of the second. It is perforated throughout so as to admit a current of air to pass through it, and the space between this tube and that which invirons it contains the oil. An ingenious apparatus, containing a piece of cotton cloth whose longitudinal threads are much the thickest, is adapted to nearly fill the space into which the oil flows. It is so contrived that the wick may be raised or depressed at pleasure. When the wick is considerably raised it is seen of a tubular form, and by the situation of the tubes already described is accessible to the air, both by means of the central perforation and the space between the exterior and second tube. When the wick is lighted, the flame is consequently in the form of a hollow cylinder, and is exceedingly brilliant. It is rendered somewhat more bright, and perfectly steady, by adapting a glass chimney whose dimensions are nearly the same with that of the exterior tube first described.
I hope this short description will be sufficient to convey an adequate idea of the instrument and shall therefore proceed to mention its effects. If the central hole be stopped, the flame changes from a cylindrical to a pyramidical form, becomes much less bright, and emits a considerable quantity of smoke. If the whole aperture be entirely or nearly stopped and the combustion becomes still more imperfect. The access of air to the external and internal surfaces of the flame is of so much importance, that a sensible difference is perceived when the hand or any other flat substance is held even at the distance of an inch from the lower aperture. There is a certain length of wick at which the effect of the lamp is the best. If the wick be too much depressed, the flame, though white and brilliant, is short; if it be raised, the flame becomes longer, and consequently the light more intense and vivid. A greater increase of the length, increases the quantity of the light, but at the same time the upper part of the flame assumes a brown hue, and smoke is emitted.
The lamp was filled with oil and weighed, it was then lighted and suffered to burn so as to produce the greatest quantity of light without smoke. After burning one hour and fifty-two minutes, it was extinguished and found to have lost 589 grains of its weight. Now a pint of oil weights 6520 grains and costs sixpence three farthings in retail; the lamp therefore consumes oil to the value of one penny in three hours. It remains to be shewn at what rate per hour the same quantity of light might be obtained from the tallow candles commonly used in families.
The candle called a middling fix, weighing upon an average the sixth part of a pound of avoirdupois, is 10¾ inches long, and 2 inches and 6/10 inch circumference. I have chosen to make my comparison with this candle as being, I imagine, most commonly used. It is to be understood that the lamp gave its maximum light without smoke.
The best method of comparing two lights with each other, that I know of, is this: Place the greater light at a considerable distance from a white paper, the less light may be moved nearer or father from the paper, accordingly as the experiment requires. If now an angular body, as the most convenient figure, be held before the paper it will project two shadows, these two shadows can coincide only in part, and their angular extremities will in all positions but one be at some distance from each other: the shadows being made to coincide in a certain part of their magnitude, they will be bordered with a lighter shadow, occasioned by the exclusion of the light from each of the two luminous bodies respectively. These lighter shadows in fact are spaces of the white paper illuminated by the different luminous bodies, and may with the greatest ease be compared together, because at a certain point they actually touch one another. If the space illuminated by the less light appear brightest, that light is to be removed farther off; and on the contrary, if it be the most obscure, that light must be brought nearer the paper. A considerable degree of precision may be obtained by this method of judging of lights, and by this method the following comparisons were made.
The candle was suffered to burn till it wanted snuffing so much, that large lumps of coaly matter were formed on the upper part of the wick. The candle then at the distance of 24 inches gave a light equal to that of the lamp at the distance of 129 inches: from this experiment it is deduced that the light of the lamp was equal to about 28 candles.
The candle was then snuffed, and it became necessary to remove it to the distance of 67 inches, before its light was so diminished as to equal that of the lamp at the before mentioned distance of 129 inches. From this experiment it is deduced that the light of the lamp was equal to not quitefour candles fresh snuffed.
Another trial with the lamp at the distance of 131 inches and a half, and another candle of the same size at the distance of 55 inches gave the lights equal. The candle was suffered to burn for some time, but did not seem to want snuffing, yet the light of the lamp then appeared to be stronger. The candle when newlysnuffed, the distances remaining the same, appeared rather to have the advantage of the lamp. These numbers give 5 2/3 candles for the light of the lamp, and I imagine the lamp to be rather better than this upon an average, because candles are suffered to go a much longer time without snuffing, and therefore in general give less lightthan was exhibited in these trials.
Another trial with the lamp raised so as to smoke a little, and the candle wanting snuffing, though the form of the wick had not yet begun to change, gave the proportion of the lamp to the candles at about 8 to 1. We may, therefore, I resume, take six middling fixes of tallow candles as an equivalent in light to the lamp. I tried the lamp against 4 candles lighted up together, placed on a distant table with the lamp, I retired till I could just discern the letters of a printed book by the light of the candles, the lamp being covered. I then directed my assistant to intercept the light of the candles and suffer the lamp to shine on the book; the lamp was the brightest. It seemed by trials of this kind to be rather better than five candles; but I was not at that time aware of the difference of the light of tallow candles, accordingly as they have been more or less recently snuffed, and as this method does not appear capable of that degree of exactness and facility the other possesses, I did not pursue it.
From these trials, it is evident that where light beyond a certain quantity is wanted, at a given place, these lamps must be highly advantageous; for the tallow candle being of six in a pound, and burning not quite seven hours, the lamp is equivalent to a pound of these candles lighted up for seven hours. Now, the expence of the lamp for seven hours is less than two pence halfpenny, and that of the candles eight pence; and if the proportion between wax and tallow candles be attended to, it will be seen that the advantages of this lamp for illuminating a theatre are very great.
The wax candles in Covent Garden Theatre are about eighty in number in the sconces, and by estimation may be worth about 2L sterling. An equal quantity of light would be afforded by fourteen of the patent lamps; for the candles used at the theatre do not give quite so much light as a tallow candle of six in a pound. The expence of the fourteen lamps for five hours will not exceed two shillings, according to the foregoing deduction.
Mr. Argand is certainly entitled to all the honour which his talents for philosophical combination have gained; and in the present instance, his claim as an inventor ought not to be disputed, though it should appear that the principle of his lamp was known and even applied to use long ago. Everyone is acquainted with the observation of Dr. Franklin, concerning the increase of light produced by joining the flames of two candles: and double candles have actually been made for, and used by shoemakers from time immemorial. The lamp of many wicks ranged in a right line, and used by watchmakers, gives a very great light for the same reason, namely because the flame being of no considerable thickness has access to air throughout and the combustion is perfectly maintained. Whereas in a thick flame the white heat or perfect ignition extends only to a certain distance from the exterior surface. This is exemplified in a striking manner in those large flames which issue from the chimnies of furnaces. These are luminous only to a certain distance inwards, and the interior part consists of vapour, hot indeed, but not on fire, so that If paper be held in the centre of the flame by means of an iron tube passed through the exterior burning part, the paper will not be set on fire. Mr. Argand has proposed the converting a right lined wick into a circular one; whether this be an advantage or no, except so far as concerns the convenience of having a longer range of conjoined flames within a less space I was desirous of ascertaining. The result of my trials are these.
I took one of Mr. Argand’s wicks, which when cut open longitudinally will form a line at the extremity proposed to be lighted, measuring about two inches and six-tenths. This wick was placed in a brass trough so that the upper edge of the wick was held perpendicular by the straight edge of the trough into which oil was put. The wick was then lighted, and it was easy to raise or lower it above the metallic edge at pleasure, because it adhered by means of the oil to the side of the brass vessel. I thus obtained a flame in a right line equal in length to the periphery of Argand’s flame, and as is the case in that lamp, I found it easy to lengthen or shorten the flame, to cause it to smoke or burn clear as has been before mentioned. The lamp and this right lined flame were placed near each other, and at the same height, the glass chimney being taken off the former: the flames of both were adjusted so as to emit a small quantity of smoke, and their lights tried. The experiment being made by means of the shadows, as before described, their lights proved exactlythe same: but to the eye, looking at both lamps together, the intensity of Argand’s flame appeared considerably the greatest; that is to say, it dazzled more and left a stronger impression when the organ of sight was directed to some other object.
Before I made this experiment I had some expectation, that the long flame would be preferable to the circular one, because I supposed the interior surface of the circular flame, could not throw out so much light as it would have done if it had been developed and exposed. I was even inclined to imagine that the greater part of the light of Argand’s lamp is furnished by the external surface of the flame. But the equality of the lights in the circular and the right-lined flames, shews that this opinion was ill founded, and that flame is in a very high degree transparent.
I therefore directed my attention to the shadow of a lighted candle, and observed, that when the candle does not smoke, the shadow is nearly the same as if the candle were not lighted; that is to say, as if there was no flame. But, if a piece of glass be held up in the same light, it will give a shadow sufficiently sensible: it therefore intercepts more of the light than flame does. This observation accounts for the superior brightness or dazzling of Argand’s lamp. For the light which falls on a given portion of the retina of the eye from Argand’s lamp is much more dense, because it consists not only of the light from the anterior but likewise from the posterior part of the flame.
My ideas on this subject were farther confirmed by an experiment I made with the two lamps; I placed the right-lined flame in such a direction that it should not, as it did before, shine on the paper by its broad side, but in the direction of its length; the comparison of its light with that of Argand’s lamp still exhibited equality. But the long flame was then much more dazzling and bright than that of Argand. This circumstance, which though highly curious, has not, as I know of, been before noticed, at least with that attention it deserves, may be applied to many valuable purposes; one in particular occurs to me that I cannot help mentioning. It should seem that anyproportion of light may be had for microscopic purposes, by means of a long flame placed in the direction of the axis of the illuminating lense.
I tried the transparency of this long flame, placed at right angles, to the ray of Argand’s lamp; it have no shadow; but when its length was placed in the direction of the ray, it gave a shadow bordered by two broad, well defined bright lines, which I have not yet sufficiently examined to be able to give any conjecture respecting them; thought they are undoubtedly owing to some optical deviation of the rays which pass in the vicinity or through the substance of the flame.
These observations on the transparency of flame suggest an improvement of which Argand’s lamp is susceptible. Instead of one ring of flame there may be two, three or more concentric rings, with air passages between them. The inner rings will shine through the outer with more facility than the presentflame does through the glass chimney; and it is probable that the rapidity of the current of air will be increased in a high proportion between these tubes of flame, so as to increase the vehemence and quantity of the ignition, and cause more light to be emitted than would answer to the mere increase of the line of wick.
P.S. Upon looking over this paper it occurred to me, that the singular fact of the same candle that gave only one twenty-eighth part of the light of the lamp, becoming so bright on being snuffed, as to give more than one fourth of the same light it was compared with (which is seven times as bright as before) might seem erroneous or founded in mistake. I have therefore, made several other experiments with snuffed and unsnuffed candles, and am well assured that a candle, newly snuffed, gives in general eight or even nine candles that have been suffered to burn undisturbed for an hour in a still place.
#39
UCL PhD students from Ucell recreate Nicholson & Carlisle’s splitting of water with battery at Bloomsbury Festival (Video)
December 20th, 2020share this
When I was asked to participate in the 2020 Bloomsbury Festival, with the Friends of St George’s Gardens, I was delighted to have an opportunity to spread the word about William Nicholson (1753-1815) and his interesting life.
I was especially happy when the FoSGG team proposed hiring an actor to play Mr Nicholson, and had great fun writing the script … up to the point when Nicholson’s interests turned to science.
It was easy to paint a vivid picture of life at sea with the East India Company, the theatrical shenanigans with Thomas Holcroft and the revolutionary and political events of that period.
But communicating about Nicholson’s experiments and the achievement of splitting water (with his colleague Anthony Carlisle) was a different matter.
I’ll confess to being a late-bloomer when it comes to any interest in science. My younger self would be astonished to learn that one day I might be interested about the history of science – nevermind reading and writing about it. I’m certainly not equipped to talk or even demonstrate it.
But just around the corner from St George’s Gardens is University College London, which was also involved with the Bloomsbury Festival, and which happens to have an electrochemistry outreach group. Several PhD students were keen to spread the word about the future of clean energy and the potential for hydrogen fuel cells – a technology which can trace its history in a direct line to Nicholson & Carlisle’s experiment in May 1800 in the house in Soho Square. (See this guest blog from Alice Llewellyn- How the discovery of electrolysis has changed the future’s energy landscape).
The UCL team comprised Alice Llewellyn, Harry Michael, Keenan Smith and Zahra Rana as presenters, and Katrina Mazloomian as videographer. They brought the science to life in a wonderfully engaging way.
This extract from ‘In Conversation with Mr Nicholson’ is now on the Nicholson’s Journal YouTube account and shows Mr Nicholson (played by Julian Date) talking to the PhD students as they:
- recreate Nicholson & Carlisle’s splitting of water with battery;
- describe how the discovery of electrolysis is useful today;
- explain the connection with global warming and clean energy; and
- describe the hydrogen fuel cell.
This is followed by a Q&A session recorded from the online event. With questions including:
- How does the oxygen and hydrogen know to come out of separate tubes?
- Has the problem of holding and gradually releasing the hydrogen been solved?
- Fuel cells are nice, but you need electricity to generate the hydrogen. Will the UK have enough capacity to generate enough electricity from non-fossil fuels?
Thankfully our team of experts from UCL answered these questions most interestingly, and in plain English as you can see on the second of two videos from the Bloomsbury Festival.
Click here to watch:
In Conversation #2: Ucell recreate Nicholson (1753-1815) splitting water by electrolysis, May 1800
#37
Eighteenth Century Mr Nicholson launches his YouTube Channel
December 15th, 2020share this

When William Nicholson launched his Journal of Natural Philosophy, Chemistry and the Arts in 1979, part of his motivation was to speed up the transfer of scientific knowledge.
If he lived today, he would surely have embraced social media for that purpose - never lowering himself to insults or trolling!
Now he has added a Nicholson’s Journal YouTube account to his media channels, and we are able to share excerpts from ‘In Conversation with Mr Nicholson’ a performance for the Bloomsbury Festival 2020 which took place in the open air of St George’s Gardens, London where Nicholson is buried.
Directed and introduced by Ian Brown, episode one is the historical part where his biographer Sue Durrell interviews Nicholson who has returned from his grave in the gardens to talk about his life in the second half of the eighteenth century.
Nicholson is brought to life most ably by actor Julian Date, who reminisces about his life at the crossroads of Georgian arts, literature, science, and commerce, and discusses the importance of his discovery in splitting water using Volta’s battery, alongside his friend Dr. Carlisle.
The short three excerpts in this video cover:
• Working for Josiah Wedgwood in Amsterdam and at the General Chamber of Manufacturers
• Nicholson’s motivation for launching his Journal of Natural Philosophy, Chemistry and The Arts; and
• Remembering Humphry Davy and the Royal Institution and recalling the experiment with Anthony Carlisle where they split water into hydrogen and oxygen in May 1800.
This is the first of two videos from this event. Part two shows demonstrations of the experiment and discusses its implications in the quest for clean energy.
Julian Date is represented by Hilary Gagan Associates.
#36
How the discovery of electrolysis has changed the future’s energy landscape
August 25th, 2020share this

A guest blog by Alice Llewellyn from UCell, the electrochemical outreach group at UCL
Shortly after the invention of the battery in the form of a voltaic pile by Alessandro Volta in 1800, William Nicholson (1753-1815) and Anthony Carlisle (1768-1840) discovered that water can be split into its constituent elements (hydrogen and oxygen) by using electrical energy.This phenomena is termed electrolysis and is the process of using electricity to produce a chemical change. Electrolysis was a critical discovery, which shook the scientific community at the time. It directly demonstrated a relationship between electricity and chemical elements. This fact helped scientific legends – Faraday, Arrhenius, Otswald and van’t Hoff develop the basics of physical chemistry as we know them.
Fast forward to today, and we are faced with one of the greatest challenges – climate change. This effect has accelerated the search for alternative fuels and energy storage devices fin order to decarbonise the energy sector. Burning fossil fuels (coal, oil and natural gas) for energy is the main cause of climate change as it produces carbon dioxide gas which leads to a greenhouse effect and the warming of our atmosphere.
A huge contender for alternative fuels is hydrogen. Hydrogen is the most abundant element in the universe. However, it does not typically exist as itself in nature and is most commonly bonded to other molecules, such as oxygen in water (H2O). This is where electrolysis plays a key role. Electrolysis can be used to extract hydrogen from the compound which can then go on to be used as a fuel. Moreover, if a renewable source of energy is used (for example wind or solar) to provide the electricity required to split the water, then there is no carbon footprint associated with this hydrogen production.
Hydrogen can then be used in fuel cells to produce electricity. Fuel cells are electrochemical energy devices, they convert chemical energy directly into electrical energy without any combustion. The way in which a fuel cell works is in fact the reverse process of electrolysis. In a fuel cell, hydrogen is split into its protons and electrons which then react with oxygen to produce water, electricity and a little bit of heat. As the only side product of this reaction is water, fuel cells are a very clean way to produce electricity.
Energy from renewable sources (wind, solar…) is intrinsically intermittent. Depending on the season or time of the day more or less energy is produced. To make sure the supply of energy is secure and stable, energy needs to be stored when an excess is produced and later fed back into the grid when needed. Water electrolysis offers grid stabilization. When a surplus of energy is available, e.g. during the day when the sun is shining, some of this energy is used to produce hydrogen. This hydrogen can then easily be stored in tanks. Whenever more energy is needed, e.g. when it is dark, hydrogen is taken from tanks and fuelcells are used to release the energy stored in the hydrogen.
Not only can hydrogen be used for grid stabilisation, but this can also be used to transform the transport sector, which contributes to around a quarter of the UK’s greenhouse gas emissions. Fuel cell vehicles are one of the solutions that have been adopted to tackle this problem and are classed as zero-emission vehicles (only water comes out of the exhaust).
In 2019, London adopted a fleet of hydrogen-powered double decker buses – a world first! As more people start to learn about this technology, more fuel cell vehicles can be spotted on our roads.
Without the discovery of electrolysis by Nicholson and Carlisle in 1800, it might not be possible to produce pure hydrogen for these applications in such an environmentally-friendly way, making the fight against climate change a more difficult task.
*
About our guest author Alice Llewellyn
Following a masters project synthesizing and testing novel battery negative electrodes, Alice Llewellyn, started her PhD project in the electrochemical innovation lab at UCL, primarily using X-ray diffraction to study atomic lattice changes in transition metal oxide cathodes during battery degradation. Alice co-runs the electrochemical outreach group UCell.
UCell is a group of PhD and masters students based at University College London, who are passionate about hydrogen, clean technologies and electricity storage and love sharing their knowledge and experience to the general public through outreach, taking their 3 kW fuel cell stack to power stages, thermal cameras and, well, anything that needs powering! In a time of a changing energy landscape, they aim to show how these technologies are starting to become a regular feature in our everyday lives.
#30
In print at last after 150 years: 'The Life of William Nicholson, by his Son'
February 24th, 2018share this

I was extremely fortunate in having been signed by one of the leading independent publishers - Peter Owen Publishing. This was the first publisher that Iapproached (so very fortunate indeed) and is surely testament to the importance of Mr Nicholson, rather than my own humble credentials.
As the main biography is taking much longer than anticipated (see below), we decided to publish The Life of William Nicholson by his Son as a prelude - exactly 150 years after it was written in 1868. This rare manuscript has been held by the Bodleian Library since 1978.
In addition to the edited text of The Life of William Nicholson by his Son, the book also includes:
- a timeline of Nicholson's life, work and inventions;
- details of Nicholson's published works;
- Nicholson's patents and inventions;
- Nicholson's list of members of the coffee house philosophical society of the 1780s (not as complete as in Discussing Chemistry and Steam by Levere and Turner …, but indicative of Nicholson's associates at the time); and
- committee Members of the Society for Naval Architecture of 1791.
Design agency Exesios have done a super job of the cover, and there is a special treat on the inside with:
- a map of all Nicholson's known homes on (Drew's map of 1785); and
- the drawings from the 1790 cylindrical printing patent.
I'm delighted that Professor Frank James of UCL and the Royal Institution has written an afterword which focuses on Nicholson's scientific contributions. Considering his literary associates, Professor James also suggests why Nicholson was never made a member of the Royal Society, despite his many achievements.
The modern biography of William Nicholson (1753-1815)
With 110,000 words under my belt, I was beginning to think that the end was in sight on the modern biography - until I met up with Hugh Torrens, Emeritus Professor of History of Science and Technology at University of Keele, to chat about some of the civil engineering issues.
Our first meeting resulted in a very exciting list of 29 items of further research which will keep me busy researching and writing for several months.
Meanwhile, you can keep up to date with news and developments here on the blog.
#22
Invention #1 - Nicholson’s Hydrometer
January 15th, 2018share this

A hydrometer is a device for measuring a density (weight per unit volume) or specific gravity (weight per unit volume compared with water). It was also called an aerometer, a gravimeter or a densimeter.
On 1 June 1784, Nicholson wrote to his good friend Mr. J. H. Magellan with: ‘A description of a new instrument for measuring the specific gravities of bodies’.
According to Museo Galileo, hydrometers date back to Archimedes and the Alexandrian teacher Hypatia, but the second half of the nineteenth century saw the design of several types which were well-used in industry of which “the better-known models include those developed by Antoine Baumé (1728-1804) and William Nicholson (1753-1815)”.
Nicholson’s paper, which does not seem to be accompanied by a drawing, was published the following year in the Memoirs of the Manchester Literary and Philosophical Society (London: Warrington, 1785) 370–380, and can be accessed via Google Books
In the first edition of Nicholson’s A Journal of Natural Philosophy, Chemistry and the Arts, Nicholson wrote an article about the hydrometers invented by Baumé – one for spirits and one for salts - which had never been used in this country, but never mentioned his own earlier invention.
In June 1797, Nicholson published a translation of a paper that had been read in France at the National Institute by Citizen Louis Bernard Guyton de Morveau (1737-1816), and then published in the Annales de Chimie. Nicholson points out that ‘this translation is nearly verbal’ as he finds himself writing about his own invention.
Comparing Nicholson’s hydrometer with that designed by Fahrenheit which he described as ‘not fit for the hand of the philosopher’, Guyton de Morveau says:
“The form which Nicholson gave some years ago to the hydrometer of Fahrenheit, rendered it proper to measure the density of solids. At present it is very much used. It gives, with considerable accuracy, the ratio of specific gravity to the fifth decimal,water being taken as unity. … It does not appear that any better instrument need be wished for in this respect.”
Of all of Nicholson’s inventions, this one still bears his name and is called Nicholson’s hydrometer today. Examples can be found in several museums, and it is possible to purchase a modern version for use in school experiments for just a few dollars.
The Oxford Museum of the History of Science kindly showed me their Nicholson’s hydrometer from 1790.

Others can be found at:
HarvardUniversity Collection of Historical Scientific Instruments
St.Mary's College in Notre Dame, Indiana
The VirtualMuseum of the History of Mineralogy (Private collection)
Sadly, I couldn’t find a video online with a demonstration of Nicholson's hydrometer being used. If anyone knows of one, or feels the urge to produce one, I would love to share it on this website.
#20
If museum image fees are "killing art history” what hope for historians of science and commerce …
November 11th, 2017share this

Image Michal Jarmoluk on Pixabay
Well done to the group of art historians who wrote to The Times on 6 November:
“The fees charged by the UK’s national museums to reproduce images of historic paintings, prints and drawings are unjustified, and should be abolished. Such fees inhibit the dissemination of knowledge that is the very purpose of public museums and galleries. Fees charged for academic use pose a serious threat to art history: a single lecture can cost hundreds of pounds; a book, thousands.”
A full copy of the letter (and more recent developments) can be found on the website www.arthistorynews.com.
As someone who uses images as a daily basis for marketing, I am used to being able to licence stock images (photographs or drawings) from websites such as Istockphoto or Shutterstock for a reasonable fee, and was shocked to find out how much some museums wished to charge, how complicated the fee structure can be, and how inconsistent the pricing structure is across various national institutions.
Initially, I had been keen to include a large number of illustrations in my modern biography of Nicholson - hoping to bring some potentially dry scientific subjects to life - but I soon had to modify my aspirations.
By way of example, when writing this blog on Nicholson’s clock, which is in the British Museum but not on public display, I was only allowed to include the three images provided by the Museum under the Commons, Attribution-NonCommercial-ShareAlike 4.0 International licence, “an internationally recognised licence recommended by one of the Directives we are expected to follow as a public sector body.”
However, the museum did not have photographs of some interesting and unique aspects of the clock including a close up of the inscription “William Nicholson / 1797” and a side view showing the fusee mechanism.
While I was permitted to take photographs, and video, for my personal use during the visit – I was not allowed to use these on the blog, as
“… you can certainly use your own images for ‘private and non-commercial purposes’ but I’m afraid you are not permitted to publish these images.
This allows us to maintain the quality of representation of our objects, keep a record of what is used and avoid any complications regarding future copyright.
The Museum’s Visitor Regulations regarding personal photography is:
8. Film, photography and audio recording
8.1 Except where indicated by notices, you are permitted to use hand-held cameras (including mobile phones) with flash bulbs or flash units, and audio and film recording equipment not requiring a stand. You may use your photographs, film and audio recordings only for your own private and non-commercial purposes.
http://www.britishmuseum.org/pdf/2011-11-14%20Visitor%20Regulations%20FINAL.pdf
The image rights team kindly offered to “easily arrange new photography for £85 + VAT (30 day turnaround but often much faster)”. How they might incur such costs was a mystery to me, and I did not bother to ask whether this was per photograph.
This seems to go against the British Museum’s object of:
The Museum was based on the practical principle that the collection should be put to public use and be FREELY accessible.
Given that Nicholson’s clock is not on public display, one might have thought they would see the benefit of some broader exposure online – at no cost to the public purse.
In thinking about what to include in book, I am faced with this pricing structure for scholarly and academic books:
Total combined print run and download units (prices per image ex-VAT):
Up to 500: £30
501 – 1,000: £40
1,001 – 2,000: £50
2,001 – 3,000: £60
My initial plan to include up to eight images, in order to properly detail the design and mechanics, would set me back £400 if the print run is between 1,000 and 2,000. Somehow, I doubt that Neil MacGregor has this problem when choosing his next set of 100 hundred objects.
There is a big difference between the commercial value in the photograph of Nicholson’s clock’s fusee and an iconic sculpture such as the Discobolus, of which the British Museum sells replicas for £2,500.
I should think that the trustees of the British Museum would have a better understanding than most of the fact that many niche historical books have only a limited customer base, but are nonetheless extremely valuable in terms of the spread of knowledge and understanding.
#17
Nicholson’s table regulator clock at the British Museum
March 3rd, 2017share this
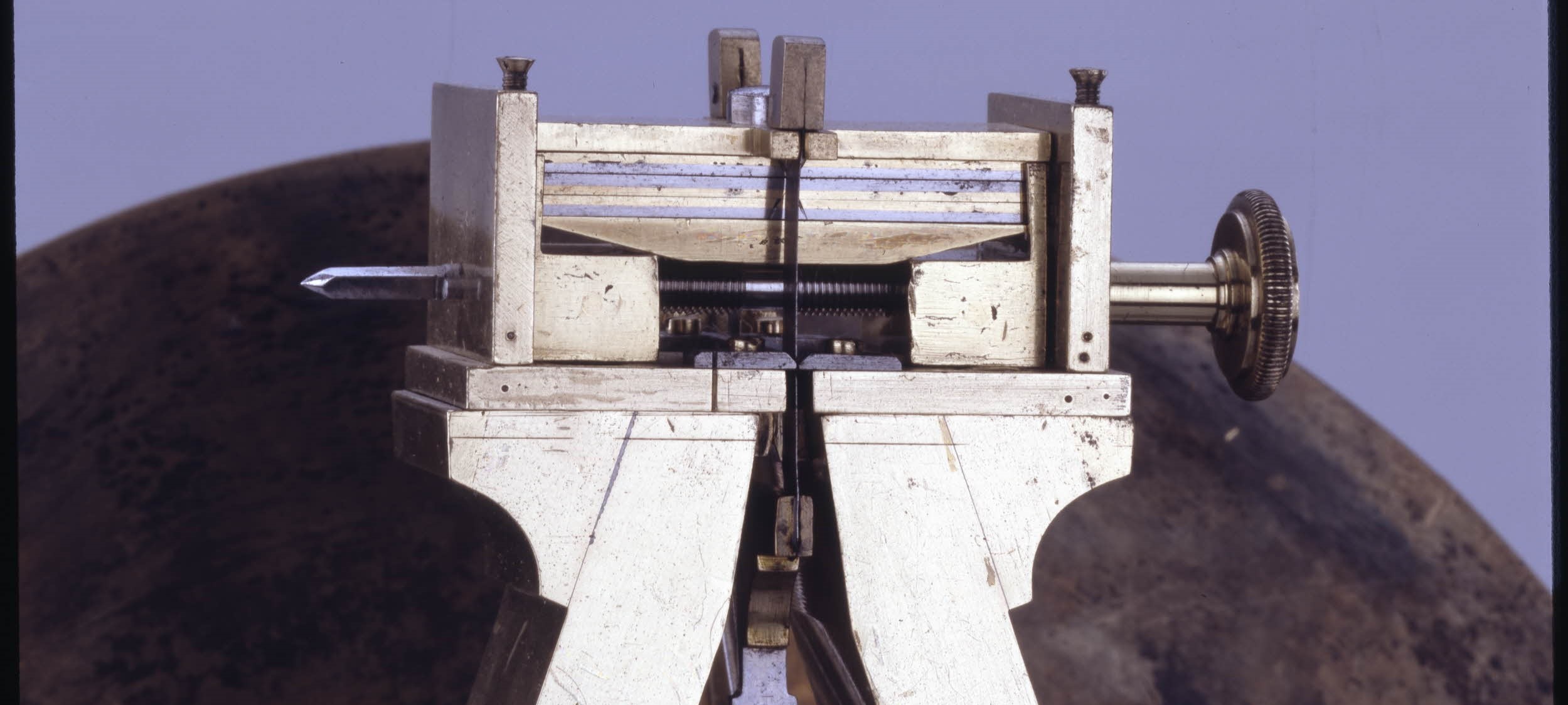
Images copyright British Museum
The British Museum was founded in 1753, the year of William Nicholson’s birth. I have evidence of at least two of his visits to the museum. The first was as part of his research for the 1783 edition of A Critical Review of the Public Buildings, Statues, and Ornaments, in and About London and Westminster. Then in January 1790, Nicholson deposited the journals of the Count de Benyowsky with the museum for safekeeping. He might have been rather delighted to know that one of his own creations would end up there too.

In 1958, one of Nicholson’s clocks was acquired by the British Museum as part of the Ilbert Collection - the most important collection of horology ever achieved by a private collector.
Courtenay Ilbert (1888-1956) was a civil engineer and he acquired the clock from a dealer called Clowes on 17 March 1938. He paid £20 for this 1797 regulator clock, a sum at the top end in comparison to the prices that Ilbert paid for other clocks from that period.
I recently enjoyed a visit behind the scenes with a curator of horology, Oliver Cooke, who had very kindly got the clock working for me. Previously I had seen the picture of the Nicholson’s clock on the British Museum website but had not registered the dimensions, and was surprised at how large it is.
Height: 57 centimetres
Width: 30.75 centimetres
Depth: 17.3 centimetres
The clock is described in David Thompson’s book Clocks as “an interesting example of a rather unassuming case which in reality conceals a movement of a most unusual and interesting design.”
The British Museum describes it as " SATINWOOD CASED EIGHT-DAY BRACKET TIMEPIECE WITH GRAVITY ESCAPEMENT AND CENTRE-SECONDS. Bracket timepiece; eight-day; gravity escapement; round silvered-metal dial with centre-seconds; satinwood case with moulded arched hood; glazed panels to front and sides surmounted by brass flaming vase finials. TRAIN-COUNT. Gt wheel 180" Click here for the full description.

The delicate mechanism for this 8-day clock rests upon a rather unprepossessing lump of steel which acts to stabilise the clock and to support the pendulum and the movement.
The plain steel pendulum rod would expand or contract with changes in temperature, but at the top Nicholson has built an ingenious mechanism with bi-metallic strips to compensate for the changes in temperature.
“Nicholson’s big solid design is going in the right direction – and it is good to see someone outside the clock-making tradition trying something different,” said Oliver Cooke. “It was clearly an attempt to make a precision timepiece, and you can see original thinking – even if Nicholson has not got it quite right.”
Nicholson’s name is engraved on the dial where the name of the commissioner/designer would usually appear: Wm Nicholson f 1797
More information can be found in:
Clocks by David Thompson, British Museum Press, London 2004.
Precision Pendulum Clocks, The Quest for Accurate Timekeeping, by Derek Roberts, Schiffer Pub Ltd, Atglen, Pennsylvania, U.S.A., 2003
#4
21stC readers of Nicholson's Journal
Can you shed light on
Mr Nicholson’s life?
Propose a guest blog

The Life of William Nicholson, 1753–1815
A Memoir of Enlightenment, Commerce, Politics, Arts and Science
Edited by Sue Durrell and with an afterword by Professor Frank James
£13.99
Order from Peter Owen Publishers
Order
